|
| 1 |
To answer most of the questions in this exercise, it will be necessary to draw the correct Lewis structure first. When bond angles or molecular shape are requested, it will be necessary to apply VSEPR theory to the Lewis structure.
The answer desired is the value predicted by VSEPR theory. For some
examples regarding bond angle, this answer will differ somewhat from the
experimental value. What is the bond order (number of bonds) in N2? |
|
|
|
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
4 |
|
|
|
None of the previous answers. |
|
| 2 |
What is the bond order (number of bonds) in I2? |
|
|
|
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
4 |
|
|
|
None of the previous answers. |
|
| 3 |
What is the OCO bond angle and shape of CO2? |
|
|
|
90o, bent |
|
|
|
109.5o, bent |
|
|
|
120o, bent |
|
|
|
180o, linear |
|
|
|
None of the previous answers. |
|
| 4 |
What is the OOO bond angle and shape of O3 (ozone)? |
|
|
|
90o, bent |
|
|
|
109.5o, bent |
|
|
|
120o, bent |
|
|
|
180o, linear |
|
|
|
None of the previous answers. |
|
| 5 |
What is the HOH bond angle and shape of H2O
according to the VSEPR model? |
|
|
|
90o, bent |
|
|
|
109.5o, bent |
|
|
|
120o, bent |
|
|
|
180o, linear |
|
|
|
None of the previous answers. |
|
| 6 |
What is the ClSCl bond angle and shape of SCl2? |
|
|
|
90o, bent |
|
|
|
109.5o, bent |
|
|
|
120o, bent |
|
|
|
180o, linear |
|
|
|
None of the previous answers. |
|
| 7 |
What are the bond angles and shape of CH2O? |
|
|
|
90o, 180o, T shaped |
|
|
|
109.5o, trigonal pyramid |
|
|
|
120o, trigonal planar |
|
|
|
180o, linear |
|
|
|
None of the previous answers. |
|
| 8 |
What are the bond angles and shape of NH3
according to the VSEPR method? |
|
|
|
90o, 180o, T shaped |
|
|
|
109.5o, trigonal pyramid |
|
|
|
120o, trigonal pyramid |
|
|
|
90o, trigonal pyramid |
|
|
|
None of the previous answers. |
|
| 9 |
What are the bond angles and shape of CH4? |
|
|
|
90o, square |
|
|
|
109.5o, tetrahedral |
|
|
|
90o, 120o, trigonal pyramid |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 10 |
What are the bond angles and shape of CHCl3? |
|
|
|
90o, square |
|
|
|
109.5o, tetrahedral |
|
|
|
90o, 120o, trigonal pyramid |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 11 |
What are the HOO bond angles in H2O2? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 12 |
What is the OSO bond angle in SO2? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 13 |
What is the HCC bond angle in C2H6? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 14 |
What is the HCC bond angle in C2H4? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 15 |
What is the HCC bond angle in C2H2? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 16 |
What is the HCN bond angle in HCN? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 17 |
What is the FNN bond angle in N2F2? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 18 |
What is the ClCO bond angle in CCl2O? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 19 |
What is the bond angle in N3-? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 20 |
What is the bond angle in ClO2-? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 21 |
What is the bond angle in ClO3-? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 22 |
What is the bond angle in NO2-? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 23 |
What is the bond angle in NO3-? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 24 |
What is the bond angle in CO32-? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 25 |
What is the bond angle in NH2-? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 26 |
What is the bond angle in NH4+? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 27 |
What is the bond angle in H3O+? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 28 |
What is the bond angle in NO2+? |
|
|
|
90o |
|
|
|
109.5o |
|
|
|
120o |
|
|
|
180o |
|
|
|
None of the previous answers. |
|
| 29 |
Oxidation states give a fairly good indication of charge when the bonding is ionic in character. However, although oxidation states are used as a bookeeping method for keeping track of electrons for covalently bonded molecules, formal charges give a more accurate indication of charge for molecules. Formal charges are also a good way of choosing between structures with different sequences of bonding. The structure with the minimal formal charges is usually the one that is right. Formal charge is calculate from f.c. = valence electrons - nonbonded electrons - bonds. What is the formal charge on the central oxygen in O3? |
|
|
|
0 |
|
|
|
1 |
|
|
|
2 |
|
|
|
-2 |
|
|
|
None of the previous answers. |
|
| 30 |
What is the formal charge on the nitrogen in NO2-? |
|
|
|
0 |
|
|
|
-3 |
|
|
|
1 |
|
|
|
5 |
|
|
|
None of the previous answers. |
|
| 31 |
What is the formal charge on the chlorine in ClO2-? |
|
|
|
-1 |
|
|
|
0 |
|
|
|
1 |
|
|
|
7 |
|
|
|
None of the previous answers |
|
| 32 |
What is the formal charge on the end nitrogens in N3-? |
|
|
|
0 |
|
|
|
1 |
|
|
|
-3 |
|
|
|
5 |
|
|
|
None of the previous answers. |
|
| 33 |
Nitrous oxide (laughing gas, N2O) could have two sequences of bonding, the usually preferred symmetrical NON and the unsymmetrical NNO. Based on the formal charges for the two sequences, which sequence (and actually is) should be the right structure? |
|
|
|
NON |
|
|
|
NNO |
|
|
|
Can' select because the formal charges are the same. |
|
|
|
|
|
|
|
|
|
| 34 |
Would the hydrogen in hypochlorous acid (HClO) be covalently bonded to the chlorine or the oxygen? |
|
|
|
chlorine |
|
|
|
oxygen |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 35 |
For the remaining problems, choose the chemical species that could be the model on the right. While most of models represent molecules, a few represent polyatomic ions.  |
|
|
|
hydrogen chloride |
|
|
|
chlorine |
|
|
|
iodine monochloride |
|
|
|
nitrogen |
|
|
|
carbon monoxide |
|
| 36 |
The model to the right could represent:  |
|
|
|
hydrogen chloride |
|
|
|
chlorine |
|
|
|
iodine monochloride |
|
|
|
nitrogen |
|
|
|
carbon monoxide |
|
| 37 |
The model to the right could represent:  |
|
|
|
hydrogen cyanide |
|
|
|
ozone |
|
|
|
nitrite |
|
|
|
water |
|
|
|
carbon dioxide |
|
| 38 |
The model to the right could represent:  |
|
|
|
hydrogen cyanide |
|
|
|
ozone |
|
|
|
nitrite |
|
|
|
water |
|
|
|
carbon disulfide |
|
| 39 |
The model to the right could represent:  |
|
|
|
hydrogen cyanide |
|
|
|
ozone |
|
|
|
nitrite |
|
|
|
water |
|
|
|
carbon dioxide |
|
| 40 |
The model to the right could represent one of the resonance structures of:  |
|
|
|
hydrogen cyanide |
|
|
|
ozone |
|
|
|
nitrite |
|
|
|
water |
|
|
|
carbon dioxide |
|
| 41 |
The model to the right could represent: 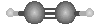 |
|
|
|
acetylene (C2H2) |
|
|
|
formaldehyde (CH2O) |
|
|
|
nitrate |
|
|
|
hydrogen peroxide (H2O2) |
|
|
|
ammonia |
|
| 42 |
The model to the right could represent: 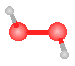 |
|
|
|
acetylene (C2H2) |
|
|
|
formaldehyde (CH2O) |
|
|
|
nitrate |
|
|
|
hydrogen peroxide (H2O2) |
|
|
|
ammonia |
|
| 43 |
The model to the right could represent one of the resonance structures of: 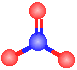 |
|
|
|
acetylene (C2H2) |
|
|
|
formaldehyde (CH2O) |
|
|
|
nitrate |
|
|
|
hydrogen peroxide (H2O2) |
|
|
|
ammonia |
|
| 44 |
The model to the right could represent:  |
|
|
|
carbon tetrachloride (CCl4) |
|
|
|
methane (CH4) |
|
|
|
chlorate |
|
|
|
thiosulfate |
|
|
|
None of the previous answers. |
|
| 45 |
The model to the right could represent: 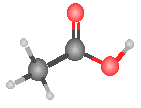 |
|
|
|
carbonic acid |
|
|
|
oxalic acid |
|
|
|
acetic acid |
|
|
|
chromic acid |
|
|
|
None of the previous answers. |
|