|
| 1 |
Questions 1 - 4 refer to the chemical equation
Zn(s) + 2 HCl(aq) = ZnCl2(aq) + H2(g)
How many moles of HCl are required for complete reaction of 0.40 moles of zinc? |
|
|
|
0.40 moles |
|
|
|
0.80 moles |
|
|
|
0.20 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 2 |
Zn(s) + 2 HCl(aq) = ZnCl2(aq) + H2(g)
For the reaction above, how many moles of hydrogen are produced from the reaction of 0.40 moles of zinc with an excess of HCl? |
|
|
|
0.40 moles |
|
|
|
0.80 moles |
|
|
|
0.20 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 3 |
Zn(s) + 2 HCl(aq) = ZnCl2(aq) + H2(g)
For the reaction above, how many moles of hydrogen are produced from the reaction of 0.40 moles of HCl with an excess of zinc? |
|
|
|
0.40 moles |
|
|
|
0.80 moles |
|
|
|
0.20 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 4 |
Zn(s) + 2 HCl(aq) = ZnCl2(aq) + H2(g)
For the reaction above, how many moles of hydrogen are produced from the reaction of 0.40 moles of Zn with an 0.40 moles of HCl? |
|
|
|
0.40 moles |
|
|
|
0.80 moles |
|
|
|
0.20 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 5 |
Questions 5 -8 refer to the chemical equation
C3H8(g) + 5 O2(g) = 3 CO2(g) + 4 H2O(g)
How many moles of O2 are required for complete reaction of 1.5 moles of propane (C3H8)? |
|
|
|
1.5 moles |
|
|
|
0.30 moles |
|
|
|
7.5 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 6 |
C3H8(g) + 5 O2(g) = 3 CO2(g) + 4 H2O(g)
For the reaction above, how many moles of carbon dioxide are produced from the reaction of 1.5 moles of propane with an excess of oxygen? |
|
|
|
4.5 moles |
|
|
|
0.50 moles |
|
|
|
1.5 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 7 |
C3H8(g) + 5 O2(g) = 3 CO2(g) + 4 H2O(g)
For the reaction above, how many moles of propane are burned when 0.60 moles of carbon dioxide are produced? |
|
|
|
1.8 moles |
|
|
|
0.2 moles |
|
|
|
0.60 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 8 |
C3H8(g) + 5 O2(g) = 3 CO2(g) + 4 H2O(g)
For the reaction above, how many moles of carbon dioxide are produced from the reaction of 0.20 moles of propane with 1.2 moles of oxygen? |
|
|
|
0.72 moles |
|
|
|
0.067 moles |
|
|
|
2.0 moles |
|
|
|
0.60 moles |
|
|
|
None of the previous answers. |
|
| 9 |
Questions 9 - 13 refer to the chemical equation
4 FeS2(s) + 11 O2(g) = 2 Fe2O3(s) + 8 SO2(g)
How many moles of O2 are required for complete reaction of 4.0x10-3 moles of FeS2? |
|
|
|
1.5x10-3 moles |
|
|
|
1.1x10-2 moles |
|
|
|
4.0x10-3 moles |
|
|
|
None of the previous answers |
|
|
|
|
|
| 10 |
4 FeS2(s) + 11 O2(g) = 2 Fe2O3(s) + 8 SO2(g)
For the reaction above, how many moles of Fe2O3 should be produced from the reaction of 4.0x10-3 moles of FeS2 with an excess of oxygen? |
|
|
|
8.0x10-3 moles |
|
|
|
4.0x10-3 moles |
|
|
|
2.0x10-3 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 11 |
4 FeS2(s) + 11 O2(g) = 2 Fe2O3(s) + 8 SO2(g)
For the reaction above, how many moles of SO2 should be produced from the reaction of 4.0x10-3 moles of FeS2 with an excess of oxygen? |
|
|
|
8.0x10-3 moles |
|
|
|
4.0x10-3 moles |
|
|
|
2.0x10-3 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 12 |
4 FeS2(s) + 11 O2(g) = 2 Fe2O3(s) + 8 SO2(g)
For the reaction above, how many moles of SO2 should be produced from the reaction of 4.0x10-3 moles of FeS2 with 8.0x10-3 moles of oxygen? |
|
|
|
1.6x10-2 moles |
|
|
|
4.0x10-3 moles |
|
|
|
1.1x10-2 moles |
|
|
|
5.8x10-3 moles |
|
|
|
None of the previous answers. |
|
| 13 |
4 FeS2(s) + 11 O2(g) = 2 Fe2O3(s) + 8 SO2(g)
For the reaction above, how many moles of FeS2 with an excess of oxygen were used to produce 3.0x10-2 moles of Fe2O3? |
|
|
|
6.0x10-2 moles |
|
|
|
3.0x10-2 moles |
|
|
|
1.50x10-2 moles |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 14 |
Questions 14 - 17 refer to the chemical equation
CH4(g) + 2 O2(g) = CO2(g) + 2 H2O(g)
How many grams of O2 are required for complete reaction of 1.6 grams of methane (CH4)? |
|
|
|
3.2 g |
|
|
|
1.6 g |
|
|
|
6.4 g |
|
|
|
0.80 g |
|
|
|
None of the previous answers. |
|
| 15 |
CH4(g) + 2 O2(g) = CO2(g) + 2 H2O(g)
For the above reaction, how many grams of water should be produced from the reaction of 1.6 grams of methane with an excess of oxygen? |
|
|
|
3.6 g |
|
|
|
3.2 g |
|
|
|
1.6 g |
|
|
|
0.90 g |
|
|
|
None of the previous answers. |
|
| 16 |
CH4(g) + 2 O2(g) = CO2(g) + 2 H2O(g)
For the above reaction, how many grams of carbon dioxide should be produced from the reaction of 1.6 grams of methane with an excess of oxygen? |
|
|
|
44 g |
|
|
|
4.4 g |
|
|
|
1.6 g |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 17 |
CH4(g) + 2 O2(g) = CO2(g) + 2 H2O(g)
For the above reaction, how many grams of methane were burned in an excess of oxygen to produce 7.2 grams of water? |
|
|
|
13 g |
|
|
|
3.6 g |
|
|
|
3.2 g |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 18 |
Questions 18 - 22 refer to the chemical equation
2 H2S(g) + SO2(g) = 3 S(s) + 2 H2O(l)
How many grams of SO2 are required for complete reaction of 1.7 grams of hydrogen sulfide? |
|
|
|
0.85 g |
|
|
|
6.4 g |
|
|
|
3.2 g |
|
|
|
1.6 g |
|
|
|
None of the previous answers |
|
| 19 |
2 H2S(g) + SO2(g) = 3 S(s) + 2 H2O(l)
How many grams of S are produced from the reaction of 1.7 grams of hydrogen sulfide with an excess of sulfur dioxide? |
|
|
|
2.4 g |
|
|
|
1.8 g |
|
|
|
1.1 g |
|
|
|
2.6 g |
|
|
|
None of the previous answers |
|
| 20 |
2 H2S(g) + SO2(g) = 3 S(s) + 2 H2O(l)
How many grams of SO2 with an excess of hydrogen sulfide were used to produce 0.75 grams of sulfur? |
|
|
|
0.25 g |
|
|
|
1.5 g |
|
|
|
0.50 g |
|
|
|
4.5 g |
|
|
|
None of the previous answers |
|
| 21 |
2 H2S(g) + SO2(g) = 3 S(s) + 2 H2O(l)
How many grams of S are produced from the reaction of 1.7 grams of hydrogen sulfide with 1.2 g of sulfur dioxide? |
|
|
|
2.4 g |
|
|
|
1.8 g |
|
|
|
2.9 g |
|
|
|
1.2 g |
|
|
|
None of the previous answers. |
|
| 22 |
2 H2S(g) + SO2(g) = 3 S(s) + 2 H2O(l)
Assume the amounts indicated in problem 21 are used and 1.2 grams of S are obtained. What is the percent yield of sulfur? |
|
|
|
100% |
|
|
|
50% |
|
|
|
67% |
|
|
|
41% |
|
|
|
None of the previous answers. |
|
| 23 |
Questions 23 - 26 refer to the chemical equation
2 Al(s) + 6 HCl(aq) = 2 AlCl3(aq) + 3 H2(g)
How many grams of HCl are required for complete reaction of 5.4 grams of aluminum? |
|
|
|
16 g |
|
|
|
2.4 g |
|
|
|
7.3 g |
|
|
|
5.4 g |
|
|
|
None of the previous answers |
|
| 24 |
2 Al(s) + 6 HCl(aq) = 2 AlCl3(aq) + 3 H2(g)
How many grams of hydrogen gas are produced from the reaction of 5.4 grams of aluminum with an excess of HCl? |
|
|
|
0.60 g |
|
|
|
0.30 g |
|
|
|
8.1 g |
|
|
|
0.27 g |
|
|
|
None of the previous answers. |
|
| 25 |
2 Al(s) + 6 HCl(aq) = 2 AlCl3(aq) + 3 H2(g)
How many grams of hydrogen gas are produced from the reaction of 5.4 grams of aluminum with 25 g of HCl? |
|
|
|
0.60 g |
|
|
|
0.30 g |
|
|
|
8.1 g |
|
|
|
0.69 g |
|
|
|
None of the previous answers. |
|
| 26 |
2 Al(s) + 6 HCl(aq) = 2 AlCl3(aq) + 3 H2(g)
Assuming the amounts of reactants given in number 25 are used and 0.50 g of hydrogen are obtained, what is the percent yield of hydrogen gas? |
|
|
|
100% |
|
|
|
83% |
|
|
|
72% |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 27 |
Questions 27 and 28 refer to the chemical equation
CaCl2(aq) + 2 AgNO3(aq) = 2 AgCl(s) + Ca(NO3)2(aq)
How many grams of AgNO3 are required for complete reaction of 0.55 grams of CaCl2? |
|
|
|
1.7 g |
|
|
|
1.1 g |
|
|
|
0.42 g |
|
|
|
0.55 g |
|
|
|
None of the previous answers. |
|
| 28 |
CaCl2(aq) + 2 AgNO3(aq) = 2 AgCl(s) + Ca(NO3)2(aq)
A 2.0 grams mixture of sodium nitrate and silver chloride is reacted with an excess of calcium chloride and 1.00 grams of silver chloride are obtained. Determine the mass percent of silver nitrate in the original mixture. |
|
|
|
50% |
|
|
|
59% |
|
|
|
118% |
|
|
|
30% |
|
|
|
None of the previous answers. |
|
| 29 |
The Haber process for the preparation of ammonia involves a combination reaction between nitrogen and hydrogen. Write a balanced molecular equation for the reaction and determine the hydrogen to nitrogen mole ratio for the reaction. |
|
|
|
1 mol H2/1 mol N2 |
|
|
|
1 mol H2/3 moles N2 |
|
|
|
3 moles H2/1 mol N2 |
|
|
|
3 moles H2/2 moles N2 |
|
|
|
None of the previous answers. |
|
| 30 |
Gasoline is a mixture of many compounds including isomers of octane (C8H18). The products of the complete combustion of gasoline are water and carbon dioxide. Write a balanced reaction for the combustion of octane and determine the optimum octane to oxygen mole ratio for the reaction. |
|
|
|
12 moles O2/1 mole C8H18 |
|
|
|
25 moles O2/2 moles C8H18 |
|
|
|
25 moles O2/1 mole C8H18 |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 31 |
The chemical equation for the reaction
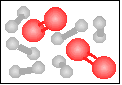 -----> -----> 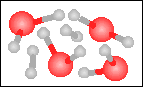 is best represented by: is best represented by:
|
|
|
|
6 H2(g) + 2 O2(g) = 4 H2O(g) + 2 H2(g) |
|
|
|
2 H2(g) + O2(g) = 2 H2O(g) |
|
|
|
H2(g) + O2(g) = H2O2(g) |
|
|
|
N2(g) + 2 O2(g) = 2 NO2(g) |
|
|
|
None of the previous answers. |
|
| 32 |
For the reaction in number 31, what was the limiting reagent? |
|
|
|
hydrogen |
|
|
|
oxygen |
|
|
|
water |
|
|
|
Neither, stoichiometric amounts were used. |
|
|
|
None of the previous answers. |
|
| 33 |
The chemical equation for the reaction
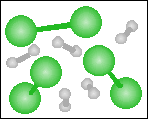 -----> -----> 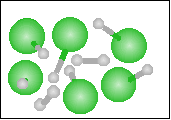 is best represented by: is best represented by:
|
|
|
|
N2(g) + O2(g) = 2 NO(g) |
|
|
|
H2(g) + O2(g) = H2O2(g) |
|
|
|
2 H2(g) + O2(g) = 2 H2O(g) |
|
|
|
H2(g) + Cl2(g) = 2 HCl(g) |
|
|
|
None of the previous answers |
|
| 34 |
For the reaction in number 33, what was the limiting reagent? |
|
|
|
hydrogen |
|
|
|
chlorine |
|
|
|
hydrogen chloride |
|
|
|
Neither, they were used in stoichiometric amounts. |
|
|
|
None of the previous answers. |
|
| 35 |
The chemical equation for the reaction
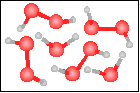 -----> ----->  is best represented by: is best represented by:
|
|
|
|
H2O2(g) = H2(g) + O2(g) |
|
|
|
4 H2O2(g) + 2 H2O(g) = 6 H2O(g) + 2 O2(g) |
|
|
|
2 H2O2(g) = 2 H2O(g) + O2(g) |
|
|
|
2 H2O(g) = 2 H2(g) + O2(g) |
|
|
|
None of the previous answers. |
|
| 36 |
Carbon monoxide reacts with hydrogen in the presence of a nickel catalyst to yield methane and water. Choose the appropriate product box for the reaction.
 -----> -----> |
|
|
|
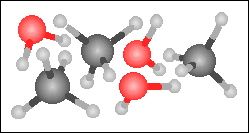 |
|
|
|
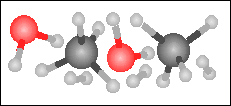 |
|
|
|
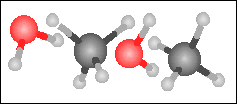 |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 37 |
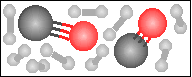
The chemical equation for the reaction
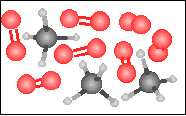 -----> -----> 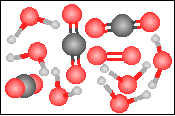 is best represented by: is best represented by:
|
|
|
|
CH4(g) + 2 O2(g) = CO2(g) + 2 H2O(g) |
|
|
|
C(s) + O2(g) = CO2(g) |
|
|
|
3 CH4(g) + 7 O2(g) = 3 CO2(g) + 6 H2O(g) + O2(g) |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 38 |
For the reaction in number 37, what was the limiting reagent? |
|
|
|
methane |
|
|
|
oxygen |
|
|
|
carbon dioxide |
|
|
|
water |
|
|
|
Neither, the reagents were used in stoichiomtric quantities. |
|
| 39 |
The chemical equation for the reaction
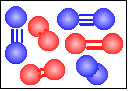 -----> -----> 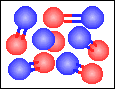 is best represented by: is best represented by:
|
|
|
|
H2(g) + O2(g) = H2O2(g) |
|
|
|
H2(g) + Cl2(g) = 2 HCl(g) |
|
|
|
3 N2(g) + 3 O2(g) = 6 NO(g) |
|
|
|
N2(g) + O2(g) = 2 NO(g) |
|
|
|
None of the previous answers. |
|
| 40 |
For the reaction in number 39, what was the limiting reagent? |
|
|
|
nitrogen |
|
|
|
oxygen |
|
|
|
nitrogen monoxide |
|
|
|
Neither, stoichiometric amounts of the reagents were used. |
|
|
|
None of the previous answers. |
|
| 41 |
The chemical equation for the reaction
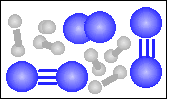 -----> -----> 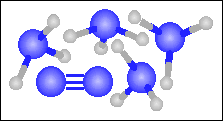 is best represented by: is best represented by:
|
|
|
|
H2(g) + O2(g) = H2O2(g) |
|
|
|
N2(g) + 3 H2(g) = 2 NH3(g) |
|
|
|
3 N2(g) + 6 H2(g) = 4 NH3(g) + N2(g) |
|
|
|
N2(g) + O2(g) = 2 NO(g) |
|
|
|
None of the previous answers. |
|
| 42 |
For the reaction in number 41 what was the limiting reagent? |
|
|
|
nitrogen |
|
|
|
hydrogen |
|
|
|
ammonia |
|
|
|
Neither, stoichiometric amounts of the reagents were used. |
|
|
|
None of the previous answers. |
|