|
| 1 |
The melting of ice is: |
|
|
|
a chemical change |
|
|
|
a heterogeneous mixture |
|
|
|
a physical change |
|
|
|
a compound |
|
|
|
none of the previous answers. |
|
| 2 |
Vinegar and oil form: |
|
|
|
a chemical change |
|
|
|
a homogeneous mixture |
|
|
|
a physical change |
|
|
|
a compound |
|
|
|
None of the previous answers. |
|
| 3 |
An unsaturated salt solution is: |
|
|
|
a heterogeneous mixture |
|
|
|
a homogeneous mixture |
|
|
|
a physical change |
|
|
|
a compound |
|
|
|
None of the previous answers. |
|
| 4 |
The decomposition of hydrogen peroxide to water and oxygen is: |
|
|
|
a chemical change. |
|
|
|
a homogeneous mixture |
|
|
|
a physical change |
|
|
|
a compound |
|
|
|
None of the previous answers. |
|
| 5 |
Density is a: |
|
|
|
an extensive physical property. |
|
|
|
a chemical property. |
|
|
|
a physical change. |
|
|
|
an intensive physical property. |
|
|
|
None of the previous answers. |
|
| 6 |
Sodium sulfate is: |
|
|
|
a homogeneous mixture. |
|
|
|
a compound |
|
|
|
a mixture of elements. |
|
|
|
a substance |
|
|
|
b and d |
|
| 7 |
Evaporation of mercury is: |
|
|
|
Mercury doesn't evaporate. |
|
|
|
a chemical change. |
|
|
|
a physical change. |
|
|
|
a heterogeneous mixture. |
|
|
|
None of the previous answers. |
|
| 8 |
What color is water? |
|
|
|
transparent |
|
|
|
clear |
|
|
|
white |
|
|
|
None of the above answers. |
|
|
|
|
|
| 9 |
Silicon is: |
|
|
|
a homogeneous mixture. |
|
|
|
an element. |
|
|
|
a compound. |
|
|
|
a physical property. |
|
|
|
None of the previous answers. |
|
| 10 |
Mass is: |
|
|
|
an extensive physical property. |
|
|
|
an intensive physical property. |
|
|
|
the same as weight. |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 11 |
Which one of the following is a compound? |
|
|
|
steel |
|
|
|
ozone |
|
|
|
cola |
|
|
|
glucose |
|
|
|
None of the previous answers. |
|
| 12 |
Chloride, sulfate, nitrate are: |
|
|
|
anions |
|
|
|
cations |
|
|
|
elements |
|
|
|
oxoanions |
|
|
|
None of the previous answers. |
|
| 13 |
Orange juice is: |
|
|
|
a homogeneous mixture |
|
|
|
a compound |
|
|
|
an unsaturated solution. |
|
|
|
a heterogeneous mixture. |
|
|
|
None of the previous answers. |
|
| 14 |
Calcium, ammonium and tin(II) ions are: |
|
|
|
anions |
|
|
|
cations |
|
|
|
elements |
|
|
|
compounds |
|
|
|
None of the previous answers. |
|
| 15 |
Solids, liquids and gases are: |
|
|
|
the only three states of matter |
|
|
|
physical properties. |
|
|
|
the three common states of matter on earth. |
|
|
|
elements |
|
|
|
None of the previous answers. |
|
| 16 |
Rusting of iron is: |
|
|
|
a physical change. |
|
|
|
a chemical change. |
|
|
|
a compound. |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 17 |
Carbon dioxide is: |
|
|
|
a mixture of elements. |
|
|
|
a homogeneous mixture. |
|
|
|
a compound |
|
|
|
a heterogeneous mixture. |
|
|
|
None of the previous answers. |
|
| 18 |
Vodka is: |
|
|
|
a homogeneous mixture. |
|
|
|
a compound. |
|
|
|
a heterogeneous mixture. |
|
|
|
an element. |
|
|
|
None of the previous answers. |
|
| 19 |
Carbonated soda is: |
|
|
|
a homogeneous mixture. |
|
|
|
an unsaturated solution |
|
|
|
a compound. |
|
|
|
a heterogeneous mixture. |
|
|
|
None of the previous answers. |
|
| 20 |
Smog is: |
|
|
|
a homogeneous mixture. |
|
|
|
an unsaturated solution |
|
|
|
a compound. |
|
|
|
a heterogeneous mixture. |
|
|
|
None of the previous answers. |
|
| 21 |
The boiling point is: |
|
|
|
an extensive physical property. |
|
|
|
an intensive physical property. |
|
|
|
a chemical change. |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 22 |
Gasoline and water are: |
|
|
|
elements. |
|
|
|
miscible. |
|
|
|
immiscible. |
|
|
|
precipitates. |
|
|
|
None of the previous answers. |
|
| 23 |
Aqueous means: |
|
|
|
it swims |
|
|
|
in water solution. |
|
|
|
without water |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 24 |
Hygroscopic and deliquescent mean: |
|
|
|
readily absorb water. |
|
|
|
hydrophobic |
|
|
|
insoluble in water |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 25 |
Sublime means: |
|
|
|
melts |
|
|
|
condenses |
|
|
|
goes directly from the solid to the gas. |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 26 |
Unsaturated solution means: |
|
|
|
more can dissolve. |
|
|
|
the solution has all the solute it can dissolve. |
|
|
|
it is a heterogenous solution. |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 27 |
Anhydrous means: |
|
|
|
contains a lot of water. |
|
|
|
without water. |
|
|
|
an unsaturated solution. |
|
|
|
a saturated solution. |
|
|
|
None of the previous answers. |
|
| 28 |
Accurate results are: |
|
|
|
reproducible. |
|
|
|
close to each other |
|
|
|
very close to the real value. |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 29 |
Precise results are: |
|
|
|
Easily measured. |
|
|
|
close to each other |
|
|
|
very close to the real value. |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 30 |
Which one of the following is a pure substance? |
|
|
|
air |
|
|
|
sea water |
|
|
|
sodium chloride |
|
|
|
aqueous hydrochloric acid |
|
|
|
None of the previous answers. |
|
| 31 |
Which of the following is a heterogeneous mixture? |
|
|
|
unsaturated salt water |
|
|
|
sodium chloride |
|
|
|
ozone |
|
|
|
water |
|
|
|
None of the previous answers. |
|
| 32 |
Which of the following is a homogeneous mixture? |
|
|
|
vodka |
|
|
|
orange juice |
|
|
|
carbonated soda |
|
|
|
smog |
|
|
|
None of the previous answers. |
|
| 33 |
For the problems that follow, figures that use models of small molecules will be used. In these models, grey represents hydrogen, black - carbon, blue - nitrogen, red - oxygen, green - fluorine and yellow represents sulfur. For the figure to the right, which one of the descriptions best describes the contents of the figure.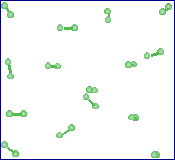 |
|
|
|
An element in its liquid state. |
|
|
|
An element in its gaseous state. |
|
|
|
A compound in the gaseous state. |
|
|
|
A homogeneous mixture. |
|
|
|
An element in its solid state. |
|
| 34 |
For the figure to the right, which one of the descriptions best describes the contents of the figure. |
|
|
|
A compound in its liquid state. |
|
|
|
A compound in its solid state. |
|
|
|
A homogeneous mixture. |
|
|
|
A heterogeneous mixture. |
|
|
|
An element in its gaseous state. |
|
| 35 |
For the figure to the right, which one of the descriptions best describes the contents of the figure. 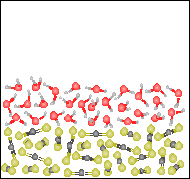 |
|
|
|
A heterogeneous mixture of two elements in their liquid states. |
|
|
|
A homogeneous mixture of two compounds in their liquid states. |
|
|
|
A heterogeneous mixture of two compounds in their liquid states. |
|
|
|
A heterogeneous mixture of two compounds in their solid states. |
|
|
|
None of the previous answers. |
|
| 36 |
Refering to the figure for the previous question (#35), which is the denser liquid, water or carbon disulfide? |
|
|
|
water |
|
|
|
carbon disulfide |
|
|
|
Not enough information to decide. |
|
|
|
|
|
|
|
|
|
| 37 |
For the figure to the right, which one of the descriptions best describes the contents of the figure.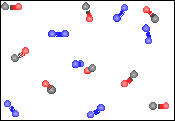 |
|
|
|
A homogeneous mixture of a compound and an element in the gaseous state. |
|
|
|
A heterogeneous mixture of a compound and an element in the gaseous state. |
|
|
|
A homogeneous mixture of a compound and an element in the liquid state. |
|
|
|
A homogeneous mixture of two elements in the gaseous state. |
|
|
|
A homogeneous mixture of two compounds in the gaseous state. |
|
| 38 |
The melting and boiling points of hydrogen fluoride are -83oC and 19oC respectively. What is the temperature of the figure to the right? 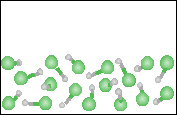 |
|
|
|
<-83oC |
|
|
|
The temperature is between -83oC and 19oC. |
|
|
|
>19oC |
|
|
|
None of the previous answers. |
|
|
|
|
|
| 39 |
For the figure in #38, which one of the descriptions best describes the contents of the figure. |
|
|
|
A compound in its solid state. |
|
|
|
A compound in its gaseous state. |
|
|
|
An element in its liquid state. |
|
|
|
A homogeneous mixture of an element and a compound. |
|
|
|
None of the previous answers. |
|